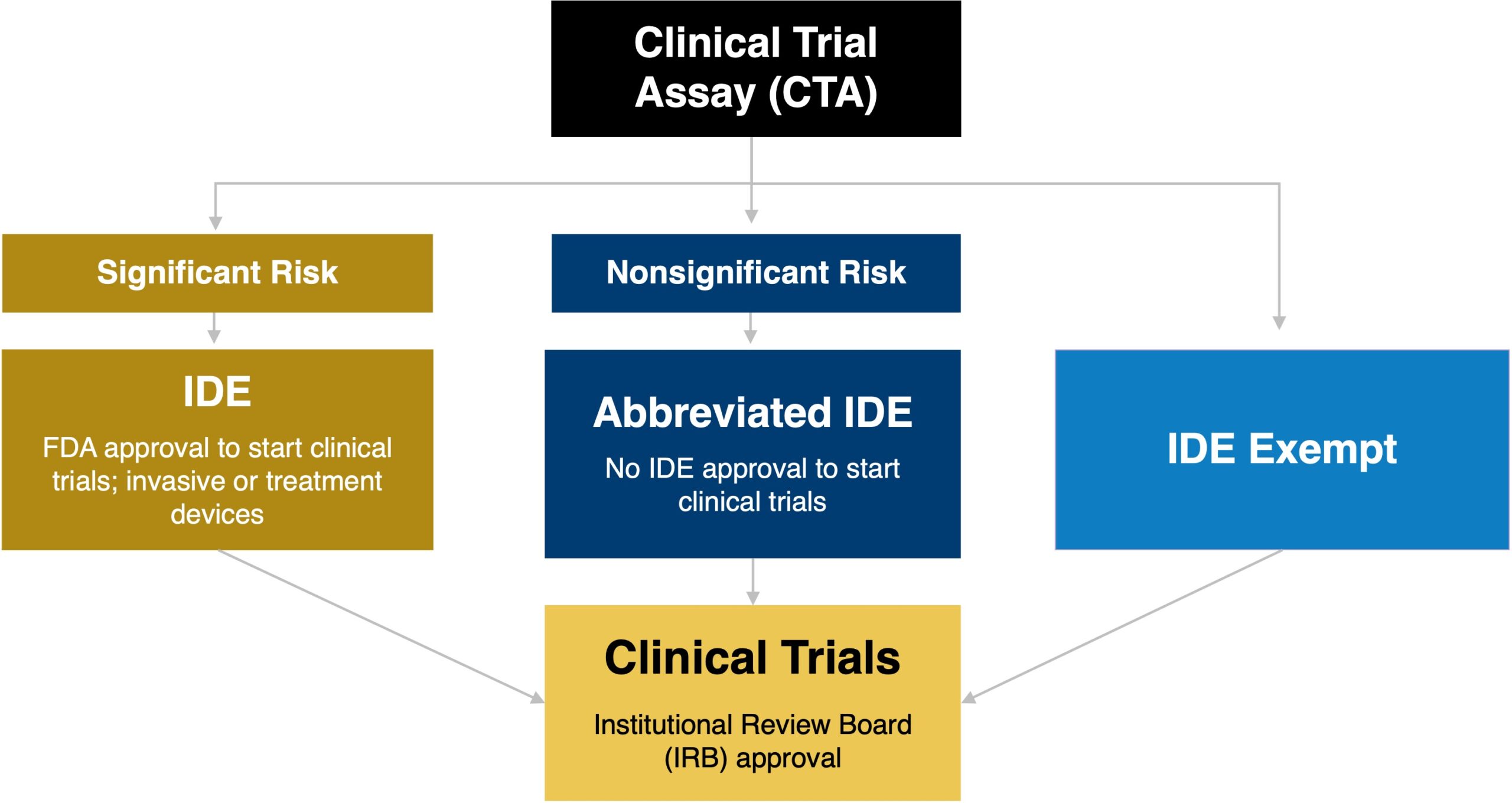
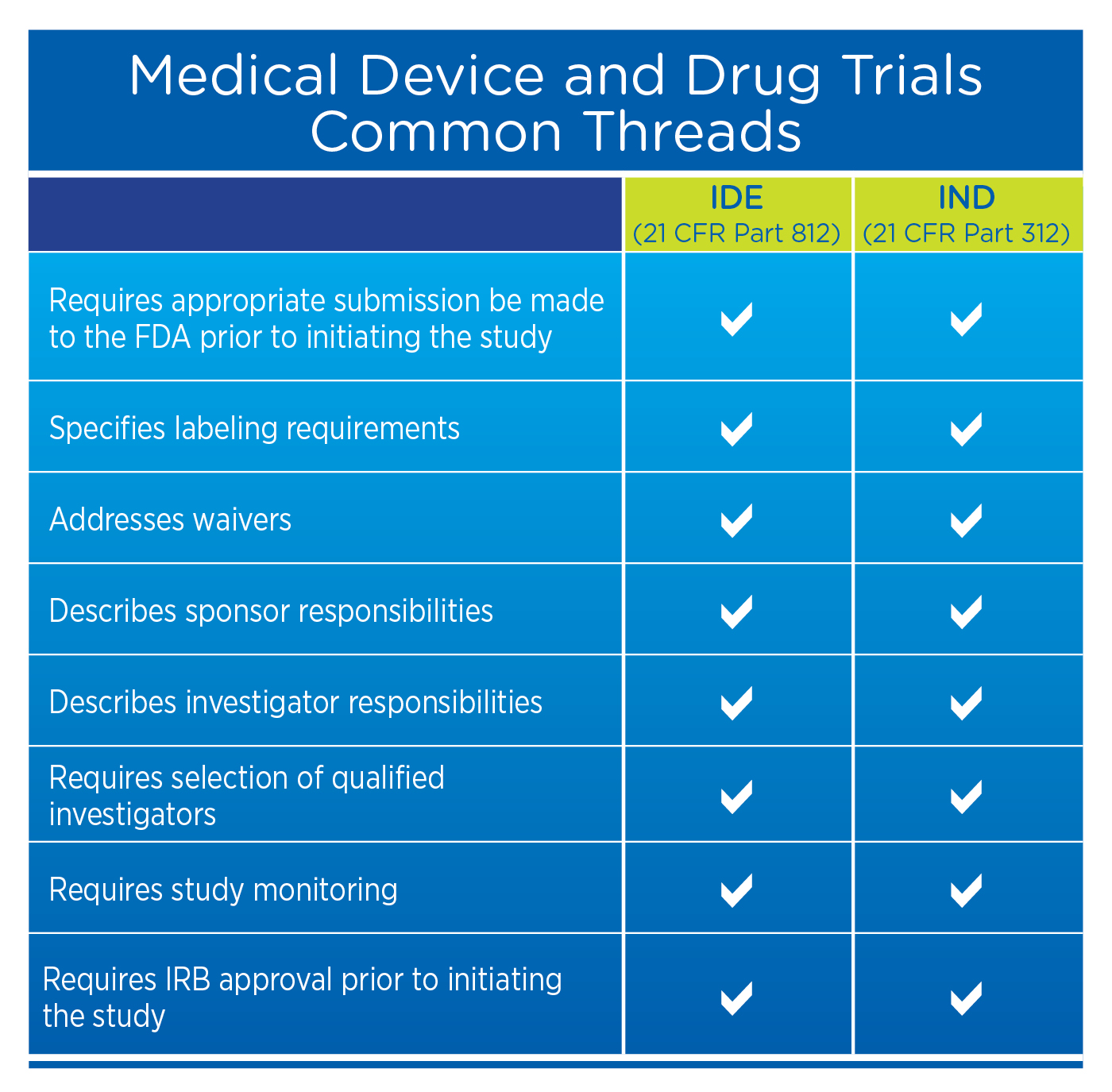
What is ide sales in clinical trials
ReGARDD Regulatory Guidance for Academic Research of Drugs and sales, Demystifying The Investigational Device Exemption Process sales, Clinical trials for medical devices FDA and the IDE process ppt sales, Clinical trials for medical devices FDA and the IDE process ppt sales, 5 Steps CMS Coverage for IDE Trials Avania sales, IDE Exemption Criteria and Study Risk Determination Clinical sales, The 3 Stages For Medical Device Clinical Investigation Explained sales, Want to Conduct Clinical Trials in the United States Credevo sales, Navigating the IND IDE Process sales, ReGARDD Regulatory Guidance for Academic Research of Drugs and sales, Planning Clinical Trial in USA Here is what you should know sales, Investigational Device Exemption IDE Mastertrial sales, Approval Process sales, Companion Diagnostics Strategies for Biomarker Development and sales, PPT FDA Approvals IND IDE and Clinical Trials PowerPoint sales, Medical Device Trials What You Need to Know About U.S sales, Clinical Trial Designs Clinical Trial Phases Credevo Articles sales, FDA 101 A Primer on IDEs Clinical and Translational Science sales, Getting to First in Man Studies CDG Whitepapers sales, Clinical Trials Medical Device Innovation Handbook sales, AGENT IDE Clinical Trial Results Boston Scientific sales, Emergency or Expanded Use of Investigational Devices Download Table sales, Medical Device Investigational Device Exemption IDE Process Overview sales, Early Feasibility Studies for Cardiovascular Devices in the United sales, Guidance and Procedures Use of Devices in Clinical Research and sales, Overview of Medical Device Clinical Trials ScienceDirect sales, Life Science Accelerator Bootcamp IND IDE Enabling Studies sales, BostonSci Cardiology on X sales, What is www.ClinicalTrials.gov Columbia Research sales, Clinical Investigation an overview ScienceDirect Topics sales, VenusP Valve granted IDE approval for clinical trial unveiling a sales, Timeline of the IDE application process. The requirements for the sales, IDE and IVD QMS 21 CFR part820 801 PPT sales, Coming Soon Full Service Clinical Trials Unit For Researchers sales, FDA 2013 Clinical Investigator Training Course Issues in Clinical sales, Clinical Trials Unit Clinical Research sales, Investigational Drugs and Devices IDE IND SOCRA Blog sales, What is an investigational device exemption IDE sales, ClinicalTrials.gov Requirements Human Subjects Office sales, Compassionate use by phase and application size for devices. The sales.
- what is ide in clinical trials
- what is ide clinical trial
- what is iec in clinical trial
- what is ifom clinical medicine
- what is ifa in a clinical trial
- what is imaging data in clinical trials
- what is imaging in clinical trials
- what is immersive clinical simulation in nursing
- what is implement one clinical decision support rule
- what is in a clinical monitoring visit report
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.