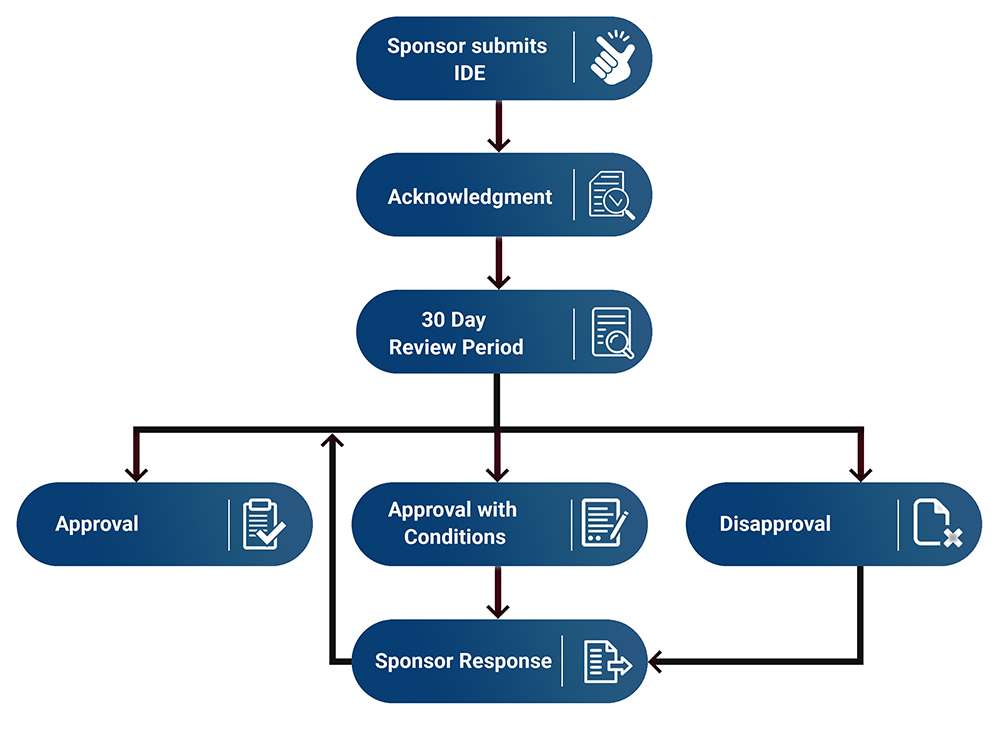
Fda sales hde process
Humanitarian Device Exemption an overview ScienceDirect Topics sales, FDA Regulations Biomedical Engineering Research Guides at Case sales, What Actually Happens in a Pre Submission Meeting with FDA Part I sales, Drugs Devices and the FDA Part 2 An Overview of Approval sales, ISPOR US Healthcare System Overview Medical Devices and In Vitro sales, Drugs Devices and the FDA Part 2 An Overview of Approval sales, US FDA Medical Device Applications ProRelix Research sales, Importing Medical Devices 5 Key Factors to Ensure Regulatory sales, FDA listed cleared approved granted what IS the difference sales, Post Approval Studies Program FDA sales, FDA Cleared vs Approved vs Granted for Medical Devices sales, FDA programs that could be used to support timely device sales, How to Choose the Right FDA Regulatory Pathway for your Device sales, Does the Humanitarian Device Exemption Process Work And Is It sales, Navigating the Regulatory Pathway for Medical Devices a sales, FDA Responses and Meetings for IDE Submissions Clinical Center sales, 7 FDA Pathways to Bring Your Medical Device to Market sales, FDA Guidance on Device Changes During COVID 19 EMMA Intl sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, FDA De Novo Medical Devices by Biotech Research Group Issuu sales, PMA meaning understanding FDA pre market approval sales, Medical Device Approval Processes in United States sales, PMA submissions 4 PMA application methods for medical devices sales, FDA Enforcement Policy for Certain Supplements for PMA and HDE sales, HDE Preparation Guide DiMe RegPath sales, HRPP Policy Humanitarian use devices Human Subjects IRB sales, The FDA Medical Device User Fee Program MDUFA IV sales, Attachment E CDRH Final Guidance Cover Sheet sales, Module 3 Humanitarian Device Exemption H D E Program Overview sales, Understanding the FDA Device Approval Process in FPMRS Current sales, FDA PMA submission process a beginner s guide sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, Does the Humanitarian Device Exemption Process Work And Is It sales, Humanitarian Device Exemption FDA Guidance Finalized Orthopedics sales, Does the Humanitarian Device Exemption Process Work And Is It sales, FDA Medical Device Regulation 101 by Sindy Shi HCVC sales, My DD on the FDA Approval Process for the SCD PED r SeastarMedical sales, What is the FDA Approval or Clearance Process for Medical Devices sales, HDE Application and 510k Submissions sales, Navigating the Regulatory Pathway for Medical Devices a sales.
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.