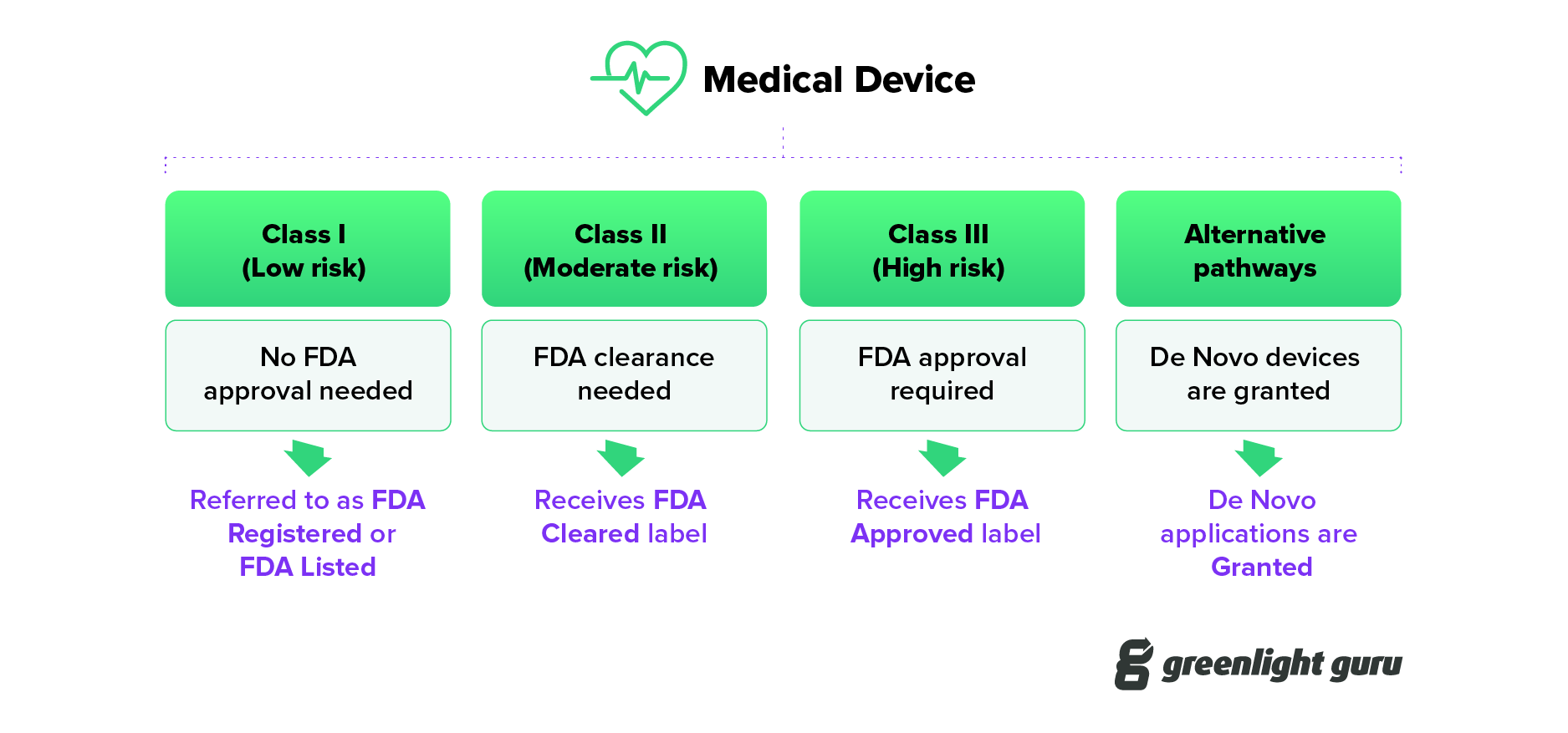
Hde sales fda definition
What IS the Relationship Between an FDA HDE Humanitarian Device sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, HDE At a Glance DiMe RegPath sales, PPT Humanitarian Use Devices HUDs Review of FDA Guidance and sales, Post Approval Studies Program FDA sales, PPT Humanitarian Use Devices HUDs Review of FDA Guidance and sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, Humanitarian Device Exemption an overview ScienceDirect Topics sales, PPT Humanitarian Use Devices HUDs Review of FDA Guidance and sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, SOLUTION Hde preparation guide dime regpath Studypool sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, PPT Humanitarian Device Exemptions HDE 101 PowerPoint sales, HDE Preparation Guide DiMe RegPath sales, Drugs Devices and the FDA Part 2 An Overview of Approval sales, FDA Enforcement Policy for Certain Supplements for PMA and HDE sales, FDA Cleared vs Approved vs Granted for Medical Devices sales, A guide to FDA regulations for medical devices Spyrosoft sales, Humanitarian Device Exemption an overview ScienceDirect Topics sales, FDA Cleared vs Approved vs Granted for Medical Devices sales, Navigating the Regulatory Pathway for Medical Devices a sales, AzCI presents Medical Device Regulations through the FDA PPT sales, The FDA Medical Device User Fee Program MDUFA IV Reauthorization sales, What IS the Relationship Between an FDA HDE Humanitarian Device sales, PPT IMDMC Regulatory 101 Pathways to Market PMA HDE De novo sales, FDA Regulation of Medical Devices EveryCRSReport sales, I. Policy A. Introduction A humanitarian use device HUD is sales, SOLUTION Module 3 humanitarian device exemption hde program sales, HDE Application and 510k Submissions sales, United States Food and Drug Administration FDA Medical Device sales, FDA Device Regulation 510 k PMA Academic Entrepreneurship for sales, Navigating the Regulatory Pathway for Medical Devices a sales, FDA Regulated Research ppt video online download sales, Humanitarian Device Exemption John Snow Labs sales, A Comprehensive Guide on eCopy Submission 510k Submission sales, FDA listed cleared approved granted what IS the difference sales, Legal Insight sales, US FDA Medical Device Applications ProRelix Research sales, Does the Humanitarian Device Exemption Process Work And Is It sales, Devices CHOP Research Institute sales.
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.