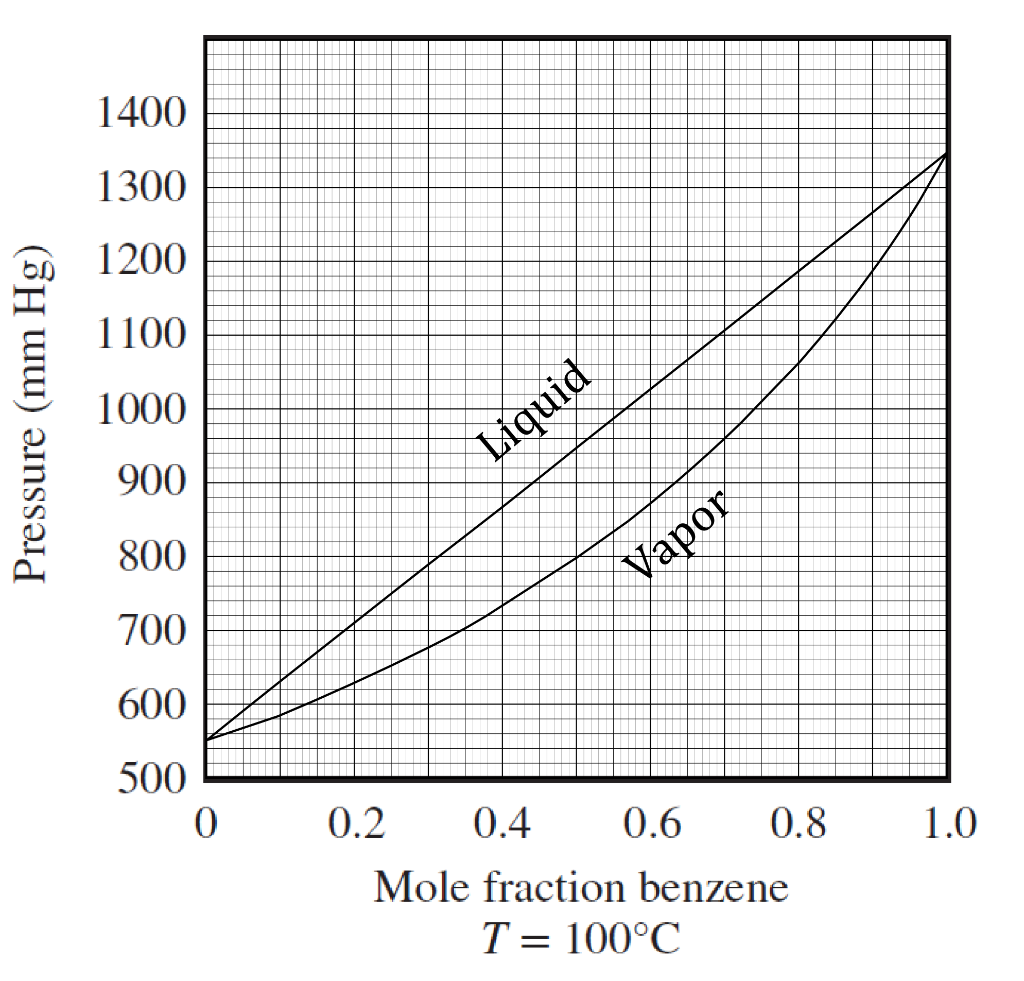
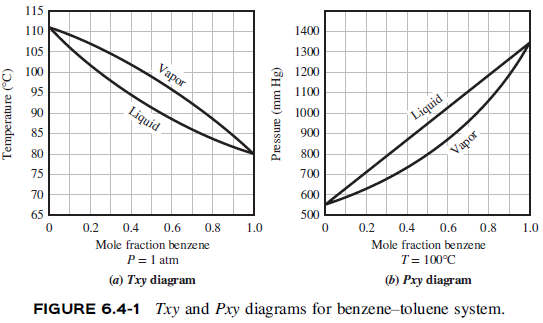
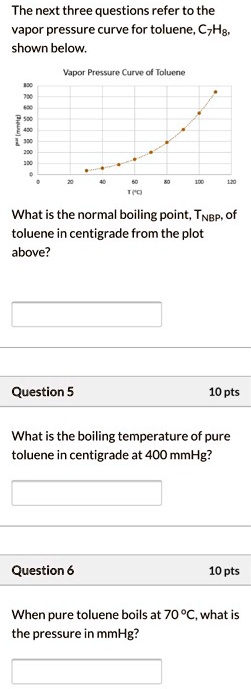
What is the boiling point sales of toluene at 600mm hg
OneClass Laboratery 7. What is the boiling polint of toluene at sales, Toluene Thermophysical Properties sales, Solved 6. Explain the effects of polarity of 7. The normal Chegg sales, Toluene Density and Specific Weight vs. Teemperature and Pressure sales, Toluene Density and Specific Weight vs. Teemperature and Pressure sales, Calculate the boiling point of water at 700mm pressure of Hg . The heat of vaporisation sales, Assume that benzene is insoluble in water and the normal boiling sales, Solved Three gram moles of benzene and 7 gram moles of Chegg sales, Solved Using Chart a what is the boiling point of a Chegg sales, E5 Solutions 04 1 Llquid Solutions Raoult s Law I Class 12th sales, The vapour pressure of pure benzene 88 oC is 957 mm and that of sales, Accuracy of vapor pressure values of toluene boiling point 110.6 sales, Solved A mixture of 6.00 gram moles of benzene and 4.00 Chegg sales, Identification of Substances by Physical Properties sales, Three gram moles of benzene and 7 gram moles of toluene are Quizlet sales, SOLVED The next three questions refer to the vapor pressure curve sales, Solved Identification of Substances by Physical Properties Chegg sales, 13. The vapour pressure of pure benzene at 25 C is 639.7 mm of Hg sales, Solved Problem 6.69 Benzene Toluene Pxy A mixture of 6.00 Chegg sales, The following graph represents variation of boiling point with sales, Equal moles of benzene and toluene are mixed the V.P. of benzene sales, Why can toluene be dried by distillation when it has almost the sales, The vapour pressure of benzene at a certain temperature is 640 mm Hg . A non volatile and sales, Water Boiling Points at Vacuum Pressure sales, Solved Problem 6.69 Benzene Toluene Pxy A mixture of 5.00 Chegg sales, 42 The vapour pressure of pure benzene at 88 C is 957 mm and sales, SOLVED The next three questions refer to the vapor pressure curve sales, Answered Four gram moles of benzene and six bartleby sales, The normal boiling point of toluene is 110.7 C and its boiling sales, The vapour pressures of benzene and toluene at 293 K are 75 mm and 22 sales, E5 Solutions 04 1 Llquid Solutions Raoult s Law I Class 12th sales, The vapour pressure of benzene and toluene at 293 K are 75 mm Hg sales, Solved Problem 6.69 Benzene Toluene Pxy A mixture of 2.00 Chegg sales, Clausius Clapeyron Equation Chemistry LibreTexts sales, Identification of Substances by Physical Properties is soluble in sales, Calculate the boiling point of water at 700mm pressure of Hg. The heat sales, Solved Problem 6.69 Benzene Toluene Pxy A mixture of 4.00 Chegg sales, SOLUTIONS . S The normal boiling point of toluene is 110.7 C sales, The vapour pressure of benzene at a certain temperature is 640 mm of Hg A sales, SOLVED 7. Pure toluene C7H8 has a normal boiling point of sales.
- what is the boiling point of toluene at 600mm hg
- what is the chemical name of hg
- what is the electronic configuration of hg
- what is the ground state shorthand notation for mercury hg
- what is the molar mass of dimethylmercury hg ch3 2
- what is the oxidation number of hg
- what is the oxidation number of hg in hg2cl2
- whats hg on the periodic table
- whats mm hg
- whatsapp hg
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.